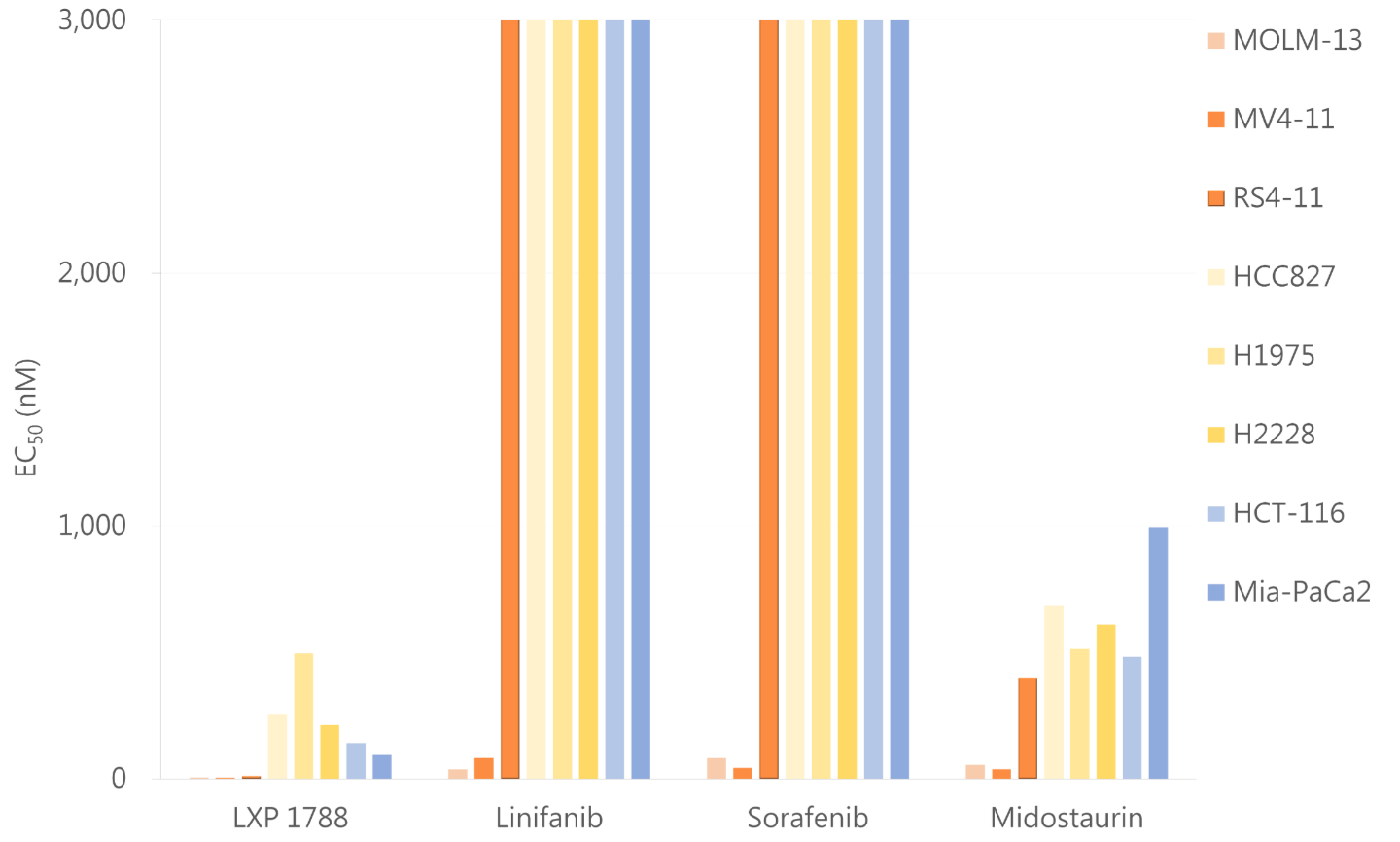
圖表顯示,LXP1788相對於其他藥物具有更好的抑制效果。
MOLM-13
Acute Monocytic Leukemia Cell Line
The cell line was established from the peripheral blood of a patient at relapse of acute monocytic leukemia which had evolved from myelodysplastic syndrome (MDS).
MV4-11
Acute Monocytic Leukemia Cell Line
The cell line was established from blasts cells of 10 years old boy with biphenotypic B-myelomonocytic leukemia.
RS4-11
Acute Monocytic Leukemia Cell Line
A lymphoblast cell line that was isolated from the marrow of a White, 32 years old, female patient with acute lymphoblastic leukemia.
H1975
Non-Small Cell Lung Cancer Cell Line
The cell line was isolated from the lungs of a nonsmoking female with non-small cell lung cancer.
H2228
Non-Small Cell Lung Cancer Cell Line
The cell line was isolated from a lung adenocarcinoma derived from a female nonsmoker with non-small cell lung cancer.
HCT-116
Colon Cancer Cell Line
The cell line was isolated from the colon of an adult male with colon cancer.
HCC827
Non-Small Cell Lung Cancer Cell Line
An epithelial cell that was isolated from the lung of a White, 39-year-old female patient with adenocarcinoma.
Mia-PaCa2
Pancreatic Cancer Cell Line
An epithelial cell line that was derived from tumor tissue of the pancreas obtained from a 65-year-old, White male.

Linifanib
- It's a inhibitor of receptor tyrosine kinase (RTK), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptor families.
- It's still on phase III clinical trial.
- Indication(s):
Hepatocellular Carcinoma

Sorafenib
- It's a inhibitor of Raf kinase, PDGF (platelet-derived growth factor), VEGF receptor 2 & 3 kinases and c Kit the receptor for Stem cell factor.
- First approved by FDA in December 20, 2005.
- Indication(s):
2005
Advanced Renal Cell Carcinoma
2007
Unresectable Hepatocellular Carcinoma
2013
Metastatic Differentiated Thyroid Cancer

Midostaurin
- It's a inhibitor of protein kinase C alpha, VEGFR2, KIT, PDGFR and WT and/or mutant FLT3 tyrosine kinases.
- First approved by FDA in April 28, 2017.
- Indication(s):
2017
FLT3-Mutated Acute Myeloid Leukemia and Systemic Mastocytosis
